Why ethnic minority groups are under-represented in breast cancer trials - and what we can do about it

A study presented at MLS Future Forum this spring investigates the underrepresentation of ethnic minority groups in breast cancer clinical trials.
The research by Ashleigh Scott was published as a paper, 'Strategies to enhance the racial and ethnic diversity of breast cancer clinical drug trials' in Academia Oncology in December 2024, and went on to win a poster prize at at the recent Minoritised Life Scientists Future Forum at the ICC in Birmingham.
Ashleigh described the significant and persistent gap in clinical cancer research as a critical issue, adding that clinical trials are the foundation of medical progress, guiding the development and approval of new treatments.
“When certain populations are systematically left out of this research, it limits our understanding of how different groups may respond to therapies, potentially leading to less effective or even harmful treatment outcomes for those not well represented,” she said.
Disparities in outcomes
“In the real world, this means that patients from ethnic minority backgrounds may not receive the same quality of care or benefit equally from new cancer treatments, contributing to disparities in survival rates and overall health outcomes.
“It matters because equitable healthcare relies on inclusive data. Without it, medical advancements may inadvertently reinforce existing inequalities rather than remedy them. Addressing this issue isn't just a matter of improving research methodology - it’s a matter of social justice and health equity.”
Ashleigh presented a bespoke strategic model, which brings together recommended strategies from the literature, highlighting actions to be first tested and then integrated alongside current initiatives to enhance trial diversity.
“In the Western world, the ‘gold standard’ route by which new efficacious therapies are tested and subsequently approved in patients is through randomised double-blinded clinical trials. Clinical trials of this kind are carried out on enrolled patients, ranging from early-phase studies on small patient numbers (<100) to late-phase international trials on several thousands of patients,” she explained.
Trial registration data
“Trial registration data should be generalisable to the wider global patient population; but very often, this is not the case, especially for common diseases affecting millions of patients such as breast cancer.
“Despite disproportionate adverse health outcomes for racial and ethnic minority (REM) patients, BC clinical trials historically show poor ethnic diversity amongst participants. In line with this, we must consider that there are many barriers to trial access, particularly for REM groups.”
Ashleigh’s research used a rapid review approach to collate successful strategies which have been used to recruit REM patients onto breast cancer clinical trials. Importantly, the rapid review also included focus group studies, capturing suggested strategies and opinions from minority groups. Seventeen papers were selected for detailed analysis following sifting.
Eight key themes arose across the selected papers and were used to compile a new Racial Minority Growth (RMG) model, shown in the accompanying figure. The model constructed here aims to portray the process of addressing the challenge of REM recruitment with multiple strategies, using puzzle pieces fitting together to create a bigger strategic picture. The overarching theme, indicated by the final papers, is that strategies must be used in a mixed-method, multi-level approach to successfully recruit REM groups.
Multifaceted challenge
“This review demonstrates that the multifaceted challenge of increasing REM recruitment in BC clinical trials needs to be tackled with multi-pronged strategies, a theme consistent across the selected studies.
“Whilst our model promotes the use of all strategies across the eight thematic areas , we note the strategies that were supported by the largest number of papers were; making cultural accommodations, community engagement, and diverse trial staff with innovative job roles such as oncology nurse navigators.”
Ashleigh said that one of the most surprising issues she came across was the lack of granular reporting when it comes to ethnicity.
“Historically there has been an obligation to record and publish trial minority data, which may be a contributing factor. Many studies tend to use broad categories like 'Black' or 'Asian' without acknowledging the vast diversity within those groups,” she said.
“For example, lumping all participants under 'Black' ignores crucial differences between Black African, Black Caribbean, African American, and other individuals of African descent - all of which may have different pharmacogenomics and hence different responses to treatment.”
Enrolment diversification
Based on the findings of the review and ensuing recommendations, RMG-inspired strategies must be implemented in hypothesis-driven randomised clinical trials to promote enrolment diversification, bolstered by quantitative data collection, Ashleigh said.
“It is imperative to make clear, practical, and evidence-based strategies that are accessible to clinical trial sponsors. Diversification needs to go deeper than clinical trials, to the core of who and what (models, criteria, policies) dictate the operation of our healthcare systems,” she pointed out.
“Future strategies need to also shift to long-term patient retention in trials to evaluate strategic sustainability. Drug regulators such as the UK MHRA should follow FDA diversity action plans, holding trial organisers accountable, and using funding to incentivise engagement.
“These collective changes have the potential to diversify trials of established breast cancer therapeutics, but also for innovative targeted treatments such as immunotherapies and individualised cellular therapies.
Model can evolve
”Our RMG model could further evolve to include a wider range of healthcare professionals, such as pharmacists aligning with reported nurse navigation models, given that pharmacists are often community-based with established patient relationships.”
The study was led by Ashleigh Scott, currently a Foundation Trainee Pharmacist. This project was her 4th-year MPharm dissertation, completed during her time at Cardiff University under the supervision of Professor Andrew Westwell. She was also supported by Bernadette Coles and Anne Cleves from the Velindre University NHS Trust library team, and Rhys Thomas from the Cardiff University School of Pharmacy and Pharmaceutical Sciences.
For more research from MLS Future Forum 2025 and updates for 2026, sign up to the newsletter.
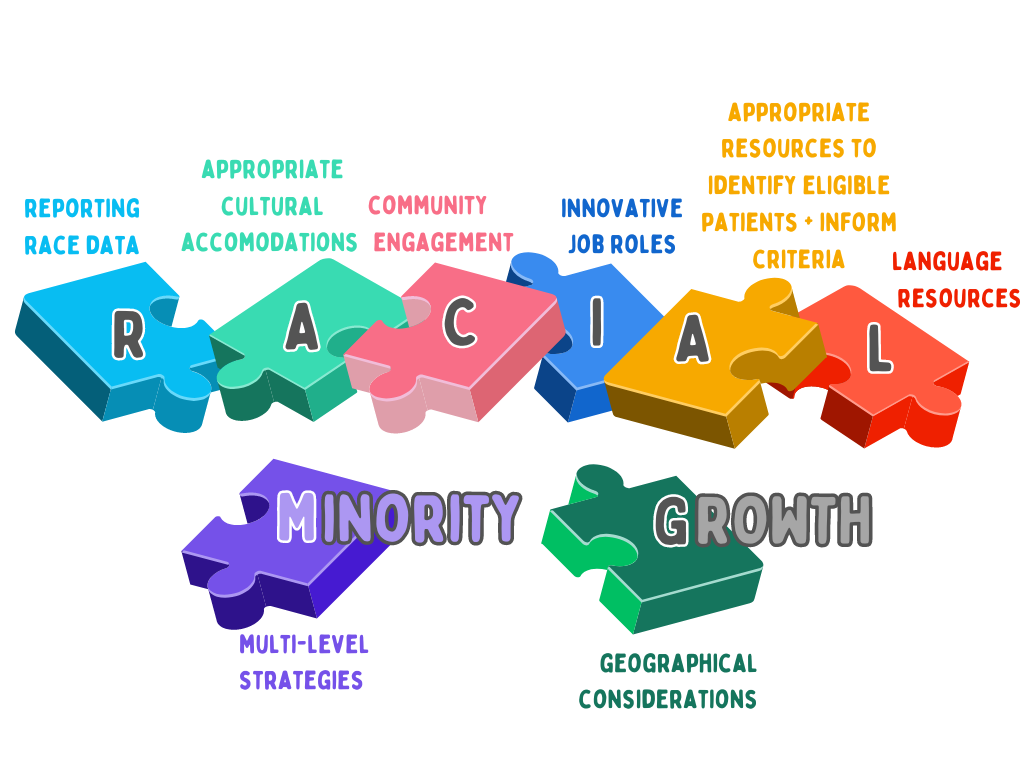
Caption: Figure 2 The Racial Minority Growth (RMG) model - a strategic framework to improve racial and ethnic minority recruitment into BC trials, bringing together key themes from the selected papers.